
Imagix 4D Version 10.5.8 Available
(San Luis Obispo/USA, Offenburg/Germany, 21 November 2025)
Imagix has released version 10.5.8 of Imagix 4D. The new version includes minor enhancements and fixes to several features, including reports, architecture views, and Delta Analysis.
Further details on the specific changes can be found in the release notes.
More information about Imagix 4D can be found here.

New Video Now Available in English: Maintaining Legacy Products made easy with Imagix 4D
(San Luis Obispo/USA, Offenburg/Germany, 8 October 2025)
Life as a software developer is tough, especially when dealing with legacy products. Time and budget pressures are constant, documentation is outdated, and the experts who once knew every detail are long gone.
Yet software bugs still need to be found fast.
Our latest video shows how Imagix 4D provides a smart and efficient solution. Already in German, this video is now also available in English, giving
international teams the chance to discover how Imagix 4D accelerates bug detection and simplifies the maintenance of complex software systems.
Watch the English version here.

Imagix 4D Version 10.5.7 Available
(San Luis Obispo/USA, Offenburg/Germany, 26 September 2025)
Imagix has released version 10.5.7 of Imagix 4D and includes extended support for current versions of Testwell CTC++.
The release contains the following changes:
- Renaming the coverage report to „Function Decision and Call Coverage“
- Using decision coverage results from Testwell CTC++ xml exports of version 9 and 10
- Enhanced matching of Testwell CTC++ probes with Imagix 4D control flow diagrams
- Improving report readability
- Fixes to coverage calculations for certain if statements
- Consistency checks of call coverage results
The „Function Decision and Call Coverage“-report contains links to the functions and source lines contributing to the results. Double clicking on links displays charts and source code for the symbols. Right clicking on function symbols lets you display flow charts of the functions where you can highlight the coverage data
More detail about specific changes is contained in the release notes.

Imagix 4D Version 10.5.6 Available
(San Luis Obispo/USA, Offenburg/Germany, 18 June 2025)
Imagix has released version 10.5.6 of Imagix 4D.
The new version adds a checklist for testing all MISRA C++:2008 rules (except chapter 14),
and introduces a checklist for AUTOSAR C++14. Additionally, source code import via Microsoft Build logs has been added.
More detail about specific changes is contained in the release notes.

Imagix 4D Version 10.5.5 Available
(San Luis Obispo/USA, Offenburg/Germany, 20 April 2025)
Imagix has released version 10.5.5 of Imagix 4D. The new version improves Delta Analysis at the Architecture Diagram level and includes extended support for current versions of Testwell CTC++.
Enhancements for Testwell CTC++ integration include:
- Support for all probe types in the updated CTC++ HTML reports
- Improved handling of Revision Branch Coverage and Accumulated Branch Coverage
- Visualization of Accumulated Branch Coverage in Control Flow Graphs
The update also contains various minor improvements and bug fixes.
More detail about specific changes is contained in the release notes.

Imagix 4D Version 10.5.4 Available
(San Luis Obispo/USA, Offenburg/Germany, 20 September 2024)
10.5.4 enhances the information generated by StackSize and VariableSize reports, expands the integration with the test coverage tool Testwell CTC++, and contains other minor enhancements and bug fixes.

Imagix 4D Version 10.5.3 Available
(San Luis Obispo/USA, Offenburg/Germany, 4 March 2024)
10.5.3 improves performance of the file editor, improves support for gcc-based code, and fixes some GUI bugs in the data sources dialog.

Imagix 4D Version 10.5.2 Available
(San Luis Obispo/USA, Offenburg/Germany, 22 December 2023)
The new version speeds up the generation of html documents by adding a concurrent (multi-threaded) mode as an alternative.

Imagix 4D Version 10.5.1 Available
(San Luis Obispo/USA, Offenburg/Germany, 23 May 2023)
Imagix 10.5.1 provides a checklist for AUTOSAR-C++ 2014 which specifies coding guidelines for the usage of the C++14 language as defined by ISO/IEC 14882:2014, in the safety-related and critical systems. The main application sector is automotive, but it can be used in other embedded application sectors. The Imagix checklist includes all rules defined by AUTOSAR.
Using the Imagix review feature guides the user in checking the rules and automates most computations. The rules requiring documentation by the user have no automated steps but ask to attach the documentation. Automated rules list all violations accurately as long as the project was analyzed without any errors and the code was complete. Rules that are partially or non-automated might still enumerate any potential locations and require human review to decide on concerns and violations. Check out the complete list of rules and their automation level.
Using AUTOSAR-C++ in Imagix requires the MISRA license extension.
Imagix 10.5.1 adds support for running some operations of the Review Tool through batch mode commands.
The new version also adds the ability to load source code into the tool through the Microsoft Visual Studio Build logfiles.
Imagix 4D 10.5.1 completes support for C++ 2022 and provides also some bugs fixes.

Imagix 4D version 10.5 with MISRA C++ Support Available
(San Luis Obispo/USA, Offenburg/Germany, 1 February 2023)
Imagix 10.5 provides a checklist for MISRA-C++ 2008, the current version of the standard for best practices in developing safety-related embedded electronic systems and other software-intensive applications in C++. It implements all rules except for chapter 14.
Using the Imagix review feature guides the user in checking the rules of MISRA-C++ and automates any computations. The rules requiring documentation by the user have no automated steps but ask to attach the documentation. The decidable rules list all violations accurately as long as the project was analyzed without any errors and the code was complete. Rules that are undecidable enumerate any potential locations and require human review to decide on concerns and violations. Using MISRA-C++ in Imagix requires the MISRA license extension.
Imagix 10.5 also adds support for loading data from source files located in directory paths using international character sets.

Imagix 4D version 10.4 Available
(San Luis Obispo/USA, Offenburg/Germany, 8 March 2022)
Moreover, there are some further minor enhancements:
- Additional display option 'By Origin' for probes in reviews set as default.
- Displaying probes with entries from different files in the same window.
- Creating call graph from architecture includes members from selected or visible subsystems.
More details are available in the release notes.
Customers with valid maintenance can download the new version from Imagix website.

First Aid for Old Code
(Offenburg/Germany, 4 March 2022)
Manufacturers must ensure the quality of their software throughout the entire lifecycle of a product. If new functions are added to older devices, but the documentation of the software is inadequate and no one really knows the old code anymore, this task becomes problematic.Using medical device software as an example, our technical article describes a suitable procedure.
Read the full article here (PDF in English)

Imagix Corporation has released version 10.3 of Imagix 4D
(San Luis Obispo/USA, Offenburg/Germany, 5 October 2021)
Other changes compared to 10.2.0:
- Resolution of calls from Java into C/C++ via the JNI has been extended.
- Areas of GUI improvements include display of source code in flow charts, and scrolling

Imagix 4D: Your Solution for Software Problems
(Offenburg/Germany, 23 September 2021)
In average more than 50% of the time in software development is spent for maintenance. There are problems in understanding software, in modifying software, in documenting software, ... Is there a solution? Yes! Imagix 4D visualizes software and speeds understanding. Metrics and software checks improve quality - the doc engine automates document generation. Learn more about Imagix 4D in our video!
Imagix 4D: Version 10.2 Available
(San Luis Obispo/USA, Offenburg/Germany, 8 April 2021)
Particular enhancements in 10.2 include:
- Additional options in generating architectures from source code
- Improved performance working with large projects, including in Architecture Diagrams
- More automation of loading code into Imagix 4D, using Soong, Gradle and JSON build systems
- Extensible interface to load code from any build logs

Imagix 4D: Version 10.1 Available
(San Luis Obispo/USA, Offenburg/Germany, 9 October 2020)
This release has 3 main features:
- Complete HIS checklist including underlying MISRA rules and metrics covering two project versions
- Delta control flow charts that show the flow chart differences between two project versions
- Unreachable Statement report and other small report enhancements
Learn more.
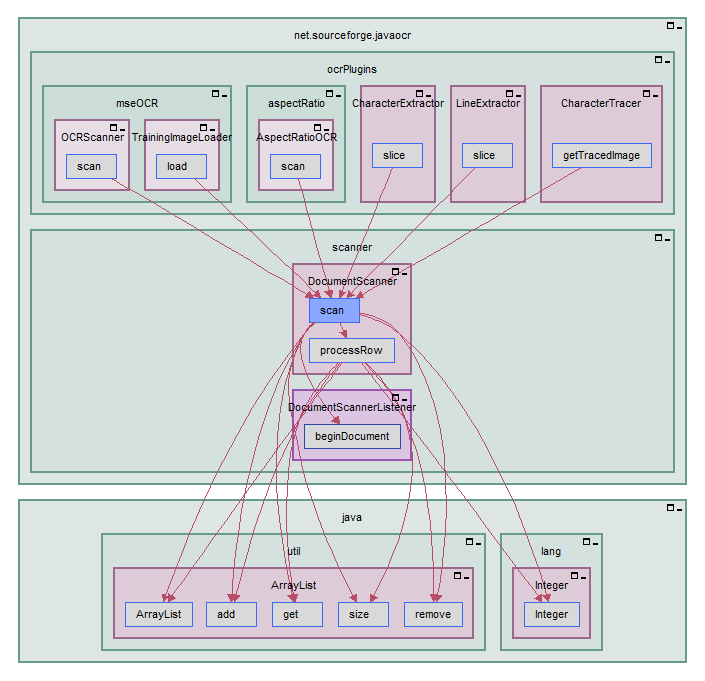
Imagix 4D: Version 10.0 Available
(San Luis Obispo/USA, Offenburg/Germany, 28 April 2020)
Version 10.0 contains a significant upgrade in the speed and lenience of the C / C++ source analyzer.
New SARIF functionality enables static analysis results from other tools to be imported and methodically reviewed within Imagix 4D, and Imagix 4D static analysis results to be exported for viewing in IDEs.
Delta Analysis had been restricted to comparing two separate projects; the storage and comparison of revisions within a project has been eliminated, in preparation for the addition of new Delta Analysis capabilities in an upcoming release.

Imagix 4D: Version 9.2 Available
(San Luis Obispo/USA, Offenburg/Germany, 30 October 2019)
It is available on Windows as well as Linux (both 32-bit and 64-bit).
Version 9.2 of Imagix 4D considerably extends the data flow analysis in reports and with data flow diagrams. These are powerful tools for pointer tracking and change analysis in projects. Sequence diagrams as an enhanced version of control flow graphs are now available in Imagix 4D.
Source code analysis is extended to the latest versions of MSVC 2019 and GCC 9 with features from C++14 and C++17. The review tool now supports the latest Common Weakness Enumeration (CWE) 3.3 checklist.
Imagix 4D Video: Software developer Anna has got a problem
(Offenburg, 15 October 2019)
Then Anna comes across the tool Imagix 4D, which determines the actual state of software based on the source code. Through the generated graphs, documentation and reports, Anna quickly identifies the causes of the errors and can fix them more easily than expected. Anna immediately informs her customers, who are more than relieved...
Watch the video now
How Imagix 4D supports the understanding of software based on source code
(Offenburg/Germany, 25 September 2019)
This work will show how Imagix 4D improves the process of exploring and understanding unfamiliar source code. An introduction into why and where such a process is required will be followed by a description of the methods Imagix 4D introduces to support such source code analysis.
Read the white paper here
Imagix has released Imagix 4D version 9.1
(Offenburg/Germany, San Luis Obispo/USA, 1 May 2018)
Imagix has released version 9.1 Imagix 4D with the following new features:
- Added metrics and analysis of stack size for embedded code
- Added import of test coverage data from Testwell CTC++
Imagix 4D releases Imagix 4D version 9.0: Introduction of a review tool and checklist for Common Weakness Enumeration (CWE)
(San Luis Obispo/USA, Offenburg/Germany, 6 march 2017)
Verifysoft Becomes First European Distributor of the Source Code Checking and Architecture Analysis Tool Imagix 4D
(Offenburg/Germany, San Luis Obispo/USA, 2 January 2016)
Imagix 4D is used for reverse-engineering, quality analysis and documentation of software written in C, C++ and Java. Customers include leading global companies like General Dynamics, Hewlett-Packard, Hitachi, Intel, Nissan, and Siemens. Imagix 4D is also used by US governmental agencies such as Federal Aviation Administration (FAA) and NASA.
Imagix is a privately-held corporation, which is headquartered in San Luis Obispo, California. > further information about Imagix
Integration of Testwell CTC++ with Imagix 4D Available
(Offenburg/Germany, San Luis Obispo/USA, 27 November 2014)
Imagix is a privately-held corporation, headquartered in San Luis Obispo, California.
> further information
